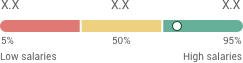سمت شغلی
شرکت
حقوق
آمار رزومههای ارسال شده
Job Description
- Develop and implement quality assurance strategies: Design and implement comprehensive quality assurance strategies, policies, and procedures that align with industry best practices and regulatory requirements. Continuously evaluate and improve existing processes to enhance overall quality and efficiency.
- Conduct quality audits: Perform regular audits and inspections to assess compliance with established quality standards. Identify areas of improvement and provide recommendations for corrective actions to ensure continuous quality enhancement.
- Create and execute quality control plans: Develop and execute detailed quality control plans and protocols to monitor the quality of products, services, and processes. Define key performance indicators (KPIs) and establish benchmarks for quality metrics.
- Monitor and analyze quality data: Collect, analyze, and interpret quality data using statistical tools and methodologies. Identify trends, patterns, and anomalies to identify potential areas of improvement or concerns. Prepare and present reports to management, highlighting key findings and recommendations.
- Collaborate with cross-functional teams: Work closely with various departments, including production, operations, and customer support, to ensure quality requirements are understood and incorporated into all aspects of the organization's activities. Provide guidance and support to team members on quality-related matters.
- Implement quality training programs: Develop and deliver training programs to enhance the quality mindset and capabilities of employees across the organization. Ensure that all employees are well-versed in quality standards, processes, and procedures.
- Manage non-conformities and corrective actions: Investigate and address non-conformities and customer complaints in a timely manner. Initiate and oversee corrective and preventive actions to rectify quality issues and prevent recurrence.
- Stay updated on industry trends and regulations: Keep abreast of industry trends, best practices, and regulatory requirements related to quality assurance. Proactively identify opportunities for process improvements and implement necessary changes to maintain compliance and exceed customer expectations.
Requirements
- Bachelor's degree in a relevant Scientific or Engineering field (e.g., Chemistry, Biochemistry). a Master’s degree is an advantage.
- At least 2 years of proven experience in quality assurance within a research and development environment.
- Ability to analyze statistical reports.
- Familiarity with laboratory and production equipment.
- Familiarity with the preparation of pharmaceutical CTD.
- Familiarity with GMP rules in the relevant assigned tasks.
Employment Type
- Full Time
Job Category
Seniority
Details
Employment type
- Full Time
Job Category
Educations
Seniority
برای مشاهدهی شغلهایی که ارتباط بیشتری با حرفهی شما دارد،
موقعیتهای شغلی مشابه
برای مشاهدهی شغلهایی که ارتباط بیشتری با حرفهی شما دارد،
کارجویان
محصولات ایرانتلنت
کارفرمایان
© 2025 IranTalent.com همه حقوق محفوظ است. شرایط و قوانین شرایط و قوانین سرویس هد هانتینگ حریم خصوصی