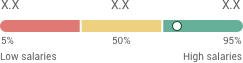Job
Company
Salary
Applicant Insights
Job Description
- To screen, review, and analyze scientific literature; generate summaries and critical assessments if appropriate.
- To Provide recommendations to marketing for scientific experts and other external parties to serve as consultants, advisory board members, or speaker-training faculty based on specific criteria.
- To present the clinical evidence to internal and external stakeholders in a non-promotional way.
- To assess the scientific accuracy and validity of non-promotion material and its compliance with the company standards, SOPs, and national laws.
- To respond to unsolicited requests from healthcare professionals. The most common requests are for clinical evidence, competitor information, updates on new data, and off-label discussions.
- To provide suitable resources and materials to healthcare professionals such as slide sets.
- To establish and maintain strong relationships with Key Opinion Leaders (KOLs).
- To identify future speakers as well as investigator sites.
- To attend relevant sessions, conduct round table discussions or advisory boards as well as collate competitor activity at congress. Also, to provide context on scientific data.
- To identify and continue liaison with potential investigators.
- To facilitate Opinion Leader input into product life cycle plans and supplementary clinical trials.
- To manage investigator-sponsored research proposals and processes.
- To provide scientific and technical support to internal colleagues.
- To provide support for research initiatives, including facilitating interactions between medical personnel and external investigators.
- To maintain the highest level of knowledge regarding current disease state products (including competitor products) and investigational compound information to enable them to be scientifically credible peers of Scientific Experts.
- To attend external national and international scientific meetings.
- To conduct training on scientific/medical information content at Product Informational Speaker Training events.
- To develop and deliver educational presentations by applicable procedures.
- To provide scientific training for sales, marketing, or other internal personnel.
- To conduct a medical review of promotional materials, on a limited
- To contribute to advocate development, and build advocacy and professional relationships for KOL engagement and KOL development.
- To collaborate as a scientific resource to actively support Medical representatives and HCPs.
- To provide medical support and training for Med Reps (Preparing educational materials).
- To ensure the pharmacovigilance process is implemented and followed.
- To follow up on clinical trial activities.
- To attend scientific events (congress, seminar, round table, and workshop) and preparation of the scientific presentation
Requirements
- MD, PharmD, PhD, or other life science qualification.
- At least 2 years of experience in the pharmaceutical field.
- Experience with clinical research, publication activities, congress presentations, and public speaking.
- Good knowledge of the international and local healthcare market, able to identify healthcare trends and related commercial opportunities.
- Strong interpersonal skills, including the capability to engage in professional relationship-building and networking.
- Ability to travel.
- Confidence and willingness to take on new challenges, contribute strategically, and manage multiple projects.
- Intellectual curiosity and intelligence about the field of science/medicine for which they are responsible.
- Ability to work effectively and share information within a team environment.
- Advanced presentation skills.
- Reliable, trustworthy, and good-tempered.
- Good knowledge of Microsoft Office, especially Outlook.
Employment Type
- Full Time
Job Category
Seniority
Details
Employment type
- Full Time
Job Category
Educations
Seniority
To see more jobs that fit your career
Similar Jobs
To see more jobs that fit your career
Candidate
Irantalent Products
Employer
© 2025 IranTalent.com All rights reserved. Terms and Conditions Head Hunting Terms & Conditions Privacy policy